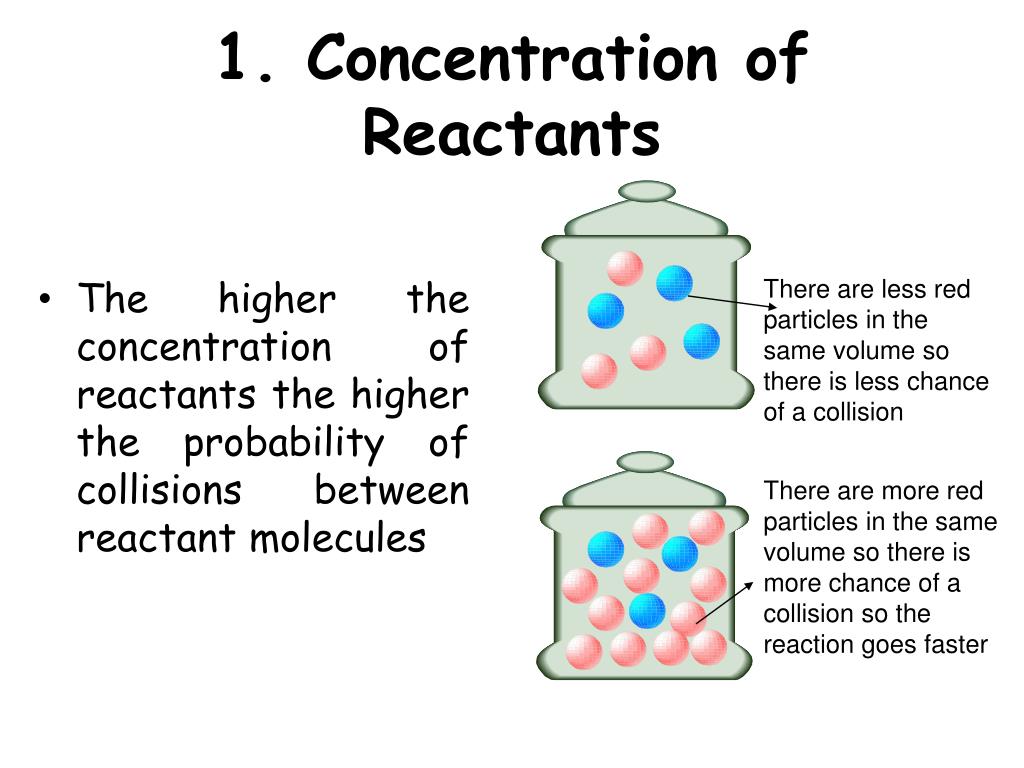
Can A Catalyst Increase The Concentration Of The Reactants . only a very small amount of catalyst is needed to increase the rate of reaction between large amounts of reactants. enzymes in the human body act as catalysts for important chemical reactions in cellular metabolism. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. suppose you are using a small amount of a solid catalyst in a reaction, and a high enough concentration of reactant in solution so that. catalysts and activation energy. As such, a deficiency of a. To increase the rate of a reaction you need to increase the number of successful collisions. an interesting property of catalysts is that they do not alter the equilibrium of the reaction (i.e. A catalyst is specific to a particular reaction:. One possible way of doing this is. describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of.
from www.slideserve.com
As such, a deficiency of a. suppose you are using a small amount of a solid catalyst in a reaction, and a high enough concentration of reactant in solution so that. catalysts and activation energy. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. One possible way of doing this is. describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of. an interesting property of catalysts is that they do not alter the equilibrium of the reaction (i.e. A catalyst is specific to a particular reaction:. enzymes in the human body act as catalysts for important chemical reactions in cellular metabolism. only a very small amount of catalyst is needed to increase the rate of reaction between large amounts of reactants.
PPT Rates of Reaction PowerPoint Presentation, free download ID3014435
Can A Catalyst Increase The Concentration Of The Reactants only a very small amount of catalyst is needed to increase the rate of reaction between large amounts of reactants. describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. catalysts and activation energy. only a very small amount of catalyst is needed to increase the rate of reaction between large amounts of reactants. suppose you are using a small amount of a solid catalyst in a reaction, and a high enough concentration of reactant in solution so that. enzymes in the human body act as catalysts for important chemical reactions in cellular metabolism. To increase the rate of a reaction you need to increase the number of successful collisions. As such, a deficiency of a. A catalyst is specific to a particular reaction:. One possible way of doing this is. an interesting property of catalysts is that they do not alter the equilibrium of the reaction (i.e.
From dxorowdpy.blob.core.windows.net
Do Catalysts Increase The Concentration Of Reactants at Daniel Glover blog Can A Catalyst Increase The Concentration Of The Reactants A catalyst is specific to a particular reaction:. an interesting property of catalysts is that they do not alter the equilibrium of the reaction (i.e. enzymes in the human body act as catalysts for important chemical reactions in cellular metabolism. One possible way of doing this is. To increase the rate of a reaction you need to increase. Can A Catalyst Increase The Concentration Of The Reactants.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Can A Catalyst Increase The Concentration Of The Reactants To increase the rate of a reaction you need to increase the number of successful collisions. suppose you are using a small amount of a solid catalyst in a reaction, and a high enough concentration of reactant in solution so that. only a very small amount of catalyst is needed to increase the rate of reaction between large. Can A Catalyst Increase The Concentration Of The Reactants.
From cemhahrt.blob.core.windows.net
How Does Catalysts Increase The Rate Of A Chemical Reaction at Gary Can A Catalyst Increase The Concentration Of The Reactants As such, a deficiency of a. only a very small amount of catalyst is needed to increase the rate of reaction between large amounts of reactants. A catalyst is specific to a particular reaction:. describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of. describe how temperatures, concentration. Can A Catalyst Increase The Concentration Of The Reactants.
From www.slideserve.com
PPT Reaction Rates 2 PowerPoint Presentation, free download ID3493687 Can A Catalyst Increase The Concentration Of The Reactants suppose you are using a small amount of a solid catalyst in a reaction, and a high enough concentration of reactant in solution so that. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. One possible way of doing this is. A catalyst is specific to a particular reaction:. an interesting property of. Can A Catalyst Increase The Concentration Of The Reactants.
From www.numerade.com
SOLVED 1 Which best defines 'reaction rate'? a) Concentration change Can A Catalyst Increase The Concentration Of The Reactants enzymes in the human body act as catalysts for important chemical reactions in cellular metabolism. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of. To increase the rate of a reaction you need to. Can A Catalyst Increase The Concentration Of The Reactants.
From chemistryguru.com.sg
Rate Concentration Graph for Enzyme Catalysed Reaction Can A Catalyst Increase The Concentration Of The Reactants enzymes in the human body act as catalysts for important chemical reactions in cellular metabolism. A catalyst is specific to a particular reaction:. One possible way of doing this is. describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of. suppose you are using a small amount of. Can A Catalyst Increase The Concentration Of The Reactants.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Can A Catalyst Increase The Concentration Of The Reactants catalysts and activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of. One possible way of doing this is. As such, a deficiency of a. A catalyst is specific to a. Can A Catalyst Increase The Concentration Of The Reactants.
From slideplayer.com
Factors Affecting the Rate of Chemical Reactions ppt download Can A Catalyst Increase The Concentration Of The Reactants describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of. suppose you are using a small amount of a solid catalyst in a reaction, and a high enough concentration of reactant in solution so that. To increase the rate of a reaction you need to increase the number of. Can A Catalyst Increase The Concentration Of The Reactants.
From dxorowdpy.blob.core.windows.net
Do Catalysts Increase The Concentration Of Reactants at Daniel Glover blog Can A Catalyst Increase The Concentration Of The Reactants suppose you are using a small amount of a solid catalyst in a reaction, and a high enough concentration of reactant in solution so that. only a very small amount of catalyst is needed to increase the rate of reaction between large amounts of reactants. enzymes in the human body act as catalysts for important chemical reactions. Can A Catalyst Increase The Concentration Of The Reactants.
From www.chegg.com
Solved Catalysts increase reaction rates by A) increasing Can A Catalyst Increase The Concentration Of The Reactants One possible way of doing this is. catalysts and activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. enzymes in the human body act as catalysts for important chemical reactions in cellular metabolism. describe how changing the temperature, concentration of a reactant, or surface area of a reaction. Can A Catalyst Increase The Concentration Of The Reactants.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Can A Catalyst Increase The Concentration Of The Reactants an interesting property of catalysts is that they do not alter the equilibrium of the reaction (i.e. suppose you are using a small amount of a solid catalyst in a reaction, and a high enough concentration of reactant in solution so that. A catalyst is specific to a particular reaction:. only a very small amount of catalyst. Can A Catalyst Increase The Concentration Of The Reactants.
From chemistryguru.com.sg
Rate Concentration Graph for Enzyme Catalysed Reaction Can A Catalyst Increase The Concentration Of The Reactants A catalyst is specific to a particular reaction:. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. only a very small amount of catalyst is needed to increase the rate of reaction between large amounts of reactants. To increase the rate of a reaction you need to increase the number of successful collisions. . Can A Catalyst Increase The Concentration Of The Reactants.
From dxorowdpy.blob.core.windows.net
Do Catalysts Increase The Concentration Of Reactants at Daniel Glover blog Can A Catalyst Increase The Concentration Of The Reactants describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of. an interesting property of catalysts is that they do not alter the equilibrium of the reaction (i.e. As such, a deficiency of a. only a very small amount of catalyst is needed to increase the rate of reaction. Can A Catalyst Increase The Concentration Of The Reactants.
From flatworldknowledge.lardbucket.org
Chemical Can A Catalyst Increase The Concentration Of The Reactants One possible way of doing this is. catalysts and activation energy. only a very small amount of catalyst is needed to increase the rate of reaction between large amounts of reactants. To increase the rate of a reaction you need to increase the number of successful collisions. describe how changing the temperature, concentration of a reactant, or. Can A Catalyst Increase The Concentration Of The Reactants.
From cemhahrt.blob.core.windows.net
How Does Catalysts Increase The Rate Of A Chemical Reaction at Gary Can A Catalyst Increase The Concentration Of The Reactants enzymes in the human body act as catalysts for important chemical reactions in cellular metabolism. As such, a deficiency of a. suppose you are using a small amount of a solid catalyst in a reaction, and a high enough concentration of reactant in solution so that. describe how temperatures, concentration of reactant, and a catalyst affect the. Can A Catalyst Increase The Concentration Of The Reactants.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog Can A Catalyst Increase The Concentration Of The Reactants describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. One possible way of doing this is. describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of. enzymes in the human body act as catalysts for important chemical reactions in cellular metabolism. As such, a. Can A Catalyst Increase The Concentration Of The Reactants.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Can A Catalyst Increase The Concentration Of The Reactants catalysts and activation energy. describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of. suppose you are using a small amount of a solid catalyst in a reaction, and a high enough concentration of reactant in solution so that. enzymes in the human body act as catalysts. Can A Catalyst Increase The Concentration Of The Reactants.
From guidelibdiapedetic.z22.web.core.windows.net
Catalyst Diagram Chemistry Can A Catalyst Increase The Concentration Of The Reactants suppose you are using a small amount of a solid catalyst in a reaction, and a high enough concentration of reactant in solution so that. only a very small amount of catalyst is needed to increase the rate of reaction between large amounts of reactants. an interesting property of catalysts is that they do not alter the. Can A Catalyst Increase The Concentration Of The Reactants.